Hydrogen
The hydrogen reaction
28 July 2005If nuclear power is to be used to generate hydrogen, suitable processes will have to be developed that will allow it to compete with existing processes. By Ken Schultz, Steve Herring, Michele Lewis and William Summers
There is currently a great deal of interest in the transition from our present petroleum-based transportation system, to one based on hydrogen. A significant ‘hydrogen economy’ is predicted that will reduce our dependence on petroleum imports whilst reducing pollution and greenhouse gas emissions. Hydrogen is an environmentally attractive fuel that has the potential to displace fossil fuels, but hydrogen is an energy carrier, not an energy source. While hydrogen is the most plentiful element on earth, all of it is chemically bound as water, hydrocarbons, carbohydrates or other compounds. Energy must be invested to separate the hydrogen.
Hydrogen is now produced for use in manufacture of fertilisers, in oil refineries to lighten heavy crude oils and produce cleaner-burning fuels, and for other industrial uses, primarily by steam reformation of methane. Worldwide production of hydrogen is about 40 million tonnes per year. The USA produces about 10 million tonnes of hydrogen a year with a thermal energy equivalent of 48GWt. In so doing, it consumes about 5% of the US natural gas usage and releases about 100 million tonnes of CO2 per year.
Transition to a hydrogen economy will require significant expansion in the production of hydrogen – by a factor of 18 more for transportation energy alone. Clearly, new sources of hydrogen will be needed. Hydrogen produced from water using nuclear energy can be one of the sources, and would avoid both use of fossil fuels and greenhouse gas emissions.
Hydrogen could be produced from nuclear energy by several means. Electricity using nuclear power can separate water into hydrogen and oxygen by electrolysis. Low temperature electrolysis is a proven, commercial technology. The net efficiency is the product of the efficiency of the reactor in producing electricity, multiplied by the efficiency of the electrolysis cell, which, at the high pressure needed for distribution and utilisation, is about 75%. For LWRs with 32% electrical efficiency, the net efficiency is about 24%. If the electrolysis were done using steam at high temperature, nuclear-produced heat could be substituted for some of the electricity and the net heat-to-hydrogen efficiency could be raised to ~50%. Thermochemical water-splitting cycles could get all of their input energy from nuclear-produced heat, using coupled, thermally-driven chemical reactions to split water into oxygen and hydrogen, and offer the promise of heat-to-hydrogen efficiencies of ~50%.
A primary source of high quality energy is needed. Nuclear power can provide that energy – and this would be a major new opportunity for nuclear.
LOW TEMPERATURE ELECTROLYSIS
Conventional or low-temperature electrolysis is a fully proven and commercial technology. About 4% of the US production of hydrogen is currently provided by this technology. Electrolysis units are commercially available from at least five major manufacturers using three different technologies – unipolar and bipolar cells using aqueous potassium hydroxide solution (alkaline electrolysis) and proton exchange membrane cells (PEM electrolysis). Large sized units use the alkaline technology. These are available in sizes up to about 2MWe (~1000kg per day of hydrogen production) and multiple units can be ganged in parallel for larger production rates. The larger units generally operate at about atmospheric pressure and the product hydrogen is then compressed to achieve pipeline pressure (20 to 30 atmospheres). High pressure electrolysis units are being developed that will make this compression unnecessary.
The net efficiency of low temperature electrolysis systems producing hydrogen at pipeline pressures is expected to reach a maximum of about 75%, measured as higher heating value (HHV) of hydrogen produced, divided by electricity used. If coupled to a LWR producing electricity at 32% thermal efficiency, low temperature electrolysis would produce hydrogen at 24% net efficiency. If coupled to an advanced reactor with 48% thermal efficiency, the net hydrogen production efficiency would be 36%. The efficiency loss and the capital cost increase in using a two-step process from heat to electricity and then electricity to hydrogen are significant penalties low temperature electrolysis must bear.
Nuclear electricity and low temperature electrolysis is used to make hydrogen today. With typical costs for electricity and electrolysis systems, however, the cost of hydrogen produced this way is high: $4-6/kg or more. Useful for small scale applications, this cannot compete with the typical $1.00-1.50/kg cost of hydrogen from natural gas.
Where large quantities of electricity are available at very low cost – for example hydroelectric plants where water would be spilled if not used – low temperature electrolysis is well suited. It can be started and stopped quickly and easily and can produce storable hydrogen when electricity is available but not needed. This might be applied to nuclear power, but only if there were large excess capacity. Today utilities meet their baseload using nuclear and other low operating cost generation, and meet their peak loads by adding fossil units. These have high fuel costs and are shutdown when not needed to meet load. If utilities could build more baseload nuclear capacity than they need and shift the output from the grid to electrolysis units during hours of low electricity demand, low temperature electrolysis might play a role in large-scale nuclear production of hydrogen.
HIGH TEMPERATURE ELECTROLYSIS
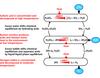
High temperature electrolysis splits steam at a temperature of about 800ºC so that hydrogen and oxygen are produced at the two electrodes, as shown in Figure 1. Operation of the cell at such high temperatures reduces the amount of electricity needed to produce a kilogram of hydrogen, since about 30% of the energy can be provided as heat rather than as electricity. In addition, at 800-1000ºC there is much lower resistance to the movement of the oxygen ions through the yttria- or scandia-stabilised zirconia electrolyte (~140µm thick) and the chemical reactions all proceed very rapidly. Finally, because the reactor is operating at high temperature, the efficiency of electricity production is much higher (about 45%). The combination of these effects could result in an overall efficiency of hydrogen production of about 45-50%.
Steam and a small amount of hydrogen (to maintain reducing conditions at the nickel-zirconia cathode) are introduced at one edge of the planar cell. Steam diffuses to the interface between the electrode and the electrolyte, where the first reaction,
2 H2O + 4 e- => 2 H2 + 2 O2-
takes place. The oxygen ions carry the electrical current through the solid electrolyte to the electrolyte–anode interface, where the reaction
2 O2- => O2 + 4 e-
occurs. The interconnect plate (below the anode in Figure 1) provides flow channels for the incoming and outgoing steam and hydrogen mixture as well as for the oxygen produced at the anode. The interconnect plate also provides the electrical connection from one cell to the next.
Oxygen flows across the lanthanum strontium manganite (LSM) electrode (anode) and the steam/hydrogen mixture flows along the nickel-zirconia cathode on the opposite side of the electrolyte.
An experimental stack of ten cells produced more than 60 normal litres of hydrogen gas per hour during extensive tests in October 2004 and January 2005.
Research at the Idaho National Laboratory (INL), in collaboration with Ceramatec, is simultaneously addressing the technical and scale-up issues associated with solid-oxide electrolysis of steam. The research includes an experimental programme aimed at performance characterisation of electrolysis cells and stacks. Results of single-cell tests have demonstrated efficient small-scale hydrogen production, with performance close to theoretical predictions. Based on these preliminary results, high-temperature electrolysis appears to be a viable means for hydrogen production using nuclear energy..
THERMOCHEMICAL WATER-SPLITTING
Thermochemical water-splitting is the conversion of water into hydrogen and oxygen by a series of thermally driven chemical reactions. Energy, as heat, is input to a thermochemical cycle via one or more endothermic high temperature chemical reactions. Heat is rejected using one or more exothermic low temperature reactions. All the reactants, other than water, are regenerated and recycled.
Sulphur-iodine cycle
General Atomics (GA), Sandia National Laboratories (SNL) and the University of Kentucky carried out a search of world literature on thermochemical water-splitting cycles. We evaluated 822 references and 115 separate thermochemical water-splitting cycles against quantifiable screening criteria and selected the 25 most promising for detailed technical evaluation. We studied the chemical
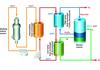
thermodynamics of these cycles and prepared preliminary engineering block flow diagrams to evaluate practicality, focusing on pure thermochemical cycles. We chose the sulphur-iodine (S-I) cycle (Figure 2).
In this cycle, iodine and sulphur dioxide are added to water, forming hydrogen iodide and sulphuric acid in an exothermic reaction. Under proper conditions, these compounds are immiscible and can be readily separated. The sulphuric acid can be decomposed at about 850°C releasing the oxygen and recycling the sulphur-
dioxide. The hydrogen iodide can be decomposed at about 350°C, releasing the hydrogen and recycling the iodine. The net reaction is the decomposition of water into hydrogen and oxygen. The whole process takes in only water and high temperature heat and releases only hydrogen, oxygen and low temperature heat. All reagents are recycled; there are no effluents.
Laboratory-scale S-I test loops at low pressure have been successfully demonstrated at the Japan Atomic Energy Research Institute (see p28). A laboratory-scale S-I cycle test loop at prototypical pressure and temperature conditions is now under construction by GA, SNL and CEA-Saclay (see Figure 3).
The S-I cycle does require high temperatures, but offers high efficiency conversion of heat energy to hydrogen (Figure 4). A major advantage is that the chemical reactions scale by volume, rather than by surface area as do electrolysis reactions. Thus economies of scale are favourable for large-scale production of hydrogen from nuclear power. Detailed design studies indicate that the S-I cycle coupled to a modular helium reactor could produce hydrogen at a cost of $1.50-2.00/kg, not much more than the current cost of hydrogen produced from natural gas, and with no emissions of CO2.
Alternate thermochemical cycles
The energy crisis of the 1970s spawned a large R&D initiative in thermochemical processes. More than 200 thermochemical cycles were proposed. While many of these cycles failed early in the development stage, a few promising ones were abandoned because interest in thermochemical cycle R&D waned with the energy crisis. As fossil fuels approach record prices, interest in thermochemical cycles has been revived. The US Department of Energy (DoE) is reconsidering some of the more promising earlier concepts as well as new concepts based on the technical advances made over the last 30 years. The DoE research programme will address alternative thermochemical processes that have been identified as having potential for high performance (high efficiency, lower temperature requirements, or reduced complexity and corrosivity) but have high technical risk.
Alternative thermochemical cycles may provide a means to bridge the technology gap associated with the baseline high-temperature sulphur cycles, which require heat at >825°C. Cycles that operate at lower temperatures (about 550°C) would have two advantages: fewer demands on materials and greater flexibility in heat sources. Nuclear reactors suitable for coupling with low temperature cycles include: advanced supercritical water reactors such as a Candu-Mark 2; an advanced sodium-cooled fast reactor; or the high temperature gas reactor (HTGR) whose highest temperature heat could then be used in a topping cycle to provide electricity.
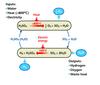
It is for these reasons that Argonne National Laboratory has been investigating the potential of a low temperature cycle, the copper-chloride (Cu-Cl) cycle.
The Cu-Cl cycle consists of four major reactions (Figure 5). Hydrogen is generated at 425°C and oxygen at 530°C. These are the highest temperatures in the cycle. Moreover, these reactions involve the generation of the gas and either a solid or liquid. They can therefore be driven to completion by simple release of the gas, thus minimising recycle flows. There are no competing reactions and, as a result, they are ideal for a cyclic process. We are now studying the intermediate reactions, which are more challenging. The combination of the relatively inexpensive chemicals and a high idealised efficiency of 49% (HHV) is attractive. While this efficiency is somewhat lower than that of the sulphur cycles, the promising chemistry leads us to believe that the Cu-Cl cycle has real potential.
The DoE is sponsoring work to identify and evaluate other immature cycles in order to ensure that no potentially important options are overlooked.
Hybrid cycles: the HyS cycle
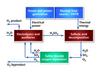
Water-splitting cycles that involve at least one electrochemical reaction step are classified as hybrid thermochemical cycles. Perhaps the best known, and most extensively studied hybrid cycle is the hybrid sulphur cycle, also known as the HyS cycle, Westinghouse Sulphur Cycle, General Atomics 22 Cycle or the Ispra Mark 11 Cycle (Figures 6 and 7). All pure thermochemical cycles (such as the sulphur-iodine process) require at least three separate reaction steps. Hybrid cycles can have just two reaction steps.
The two-step HyS cycle is:
H2SO4 => SO2 + H2O + 1/2 O2
Thermochemical, 800-900°C
2 H2O + SO2 => H2SO4 + H2
Electrochemical, 100-120°C
The presence of SO2 at the anode of the electrolyser greatly decreases the reversible cell potential needed to split water molecules by electrolysis. Whereas direct electrolysis of water has a reversible cell potential of 1.23V at 25°C, the theoretical potential for SO2 anode-depolarised electrolysis is only 0.17V per cell. Practical SO2 electrolysers are expected to operate with only 25% of the electricity needs of conventional water electrolysers. When combined with the endothermic decomposition of H2SO4, the net thermal energy requirement for the HyS cycle is significantly less than that for direct water electrolysis. The challenge is to develop the process in a way that maximises this thermal efficiency advantage yet minimises complexity and capital cost.
Recent work by the Savannah River National Laboratory (SRNL) has resulted in several process improvements to the HyS cycle. A process design and a flowsheet analysis using the AspenPlus process analyser software was conducted for a HyS system combined with a helium-cooled nuclear reactor. The overall net thermal efficiency for the plant was calculated as 48.8% based on thermal input to the process at 900°C. The efficiency was based on the HHV of the hydrogen product divided by the total thermal energy requirements, including the thermal energy used to generate electricity for the electrolysis process. Higher thermal efficiencies, exceeding 50% HHV-basis, are deemed feasible based on further optimised process flowsheets and the use of higher process operating temperatures.
Development of an SO2-depolarised electrolyser that can meet the performance goals, has a long operating lifetime and is cost effective is a major focus of ongoing research efforts. SRNL is currently developing an electrolyser based on the use of a proton-exchange membrane (PEM) electrolyte. The goal is to leverage the considerable research being performed in the field of PEM fuel cells in order to develop a low-cost, high efficiency electrolyser design. The unique conditions of SO2 electrolysis, such as the need to deal with sulphuric acid and dissolved SO2, make the development challenging.
Developing a low-cost electrolyser is a key factor in obtaining a cost-effective HyS cycle. Based on reasonable extrapolations of PEM water electrolyser cost projections, a preliminary economic analysis shows that a HyS cycle combined with a helium-cooled gas reactor could produce hydrogen at costs of approximately $1.60/kg, similar to those of the sulphur-iodine process. The inclusion of credits for by-product oxygen sales would further reduce the hydrogen production costs.
The primary technical issues to be addressed for the HyS cycle include optimisation of system operating conditions (temperature, pressure, acid concentration), selection of electrolyser materials of construction, cell design (including membrane selection and electrocatalyst loadings), and overall durability and performance. Other technical issues are associated with the sulfuric acid decomposition section of the process and the SO2/O2 separation system. An integrated laboratory-scale closed-loop demonstration of the complete HyS cycle, including both the electrolyser and acid decomposition sections, is a necessary next step.
REACTORS FOR NUCLEAR PRODUCTION OF HYDROGEN
Low-temperature electrolysis uses only water and electricity as inputs. Thus hydrogen production using low-temperature electrolysis is decoupled from the reactor and could even be located separately. High-temperature electrolysis and thermochemical water-splitting, however, receive part or all of their energy input as heat directly from the reactor. They are closely coupled to and must be located near the reactor. For these systems an intermediate heat transport loop is placed between the reactor coolant loop and the hydrogen production system. This assures that any leakage from the reactor coolant loop will not contaminate the hydrogen production system or expose hydrogen plant personnel to radiation from the primary loop coolant. We believe the hydrogen production plant can be a non-nuclear facility. The intermediate loop also ensures that the hydrogen process fluids cannot enter the core of the nuclear reactor. The heat exchanger interface sets the boundary conditions for selection of the reactor system. For example, the temperature requirement for high temperature electrolysis, the sulphur-iodine cycle or the hybrid sulphur cycle are all about 800-900°C. This must account for the temperature drop between the core outlet and the point of application in the hydrogen production system. So a reactor outlet temperature of about 950°C is needed. If successful, lower temperature thermochemical cycles such as the Cu-Cl cycle may allow lower temperatures from the reactor.
SNL evaluated various nuclear reactors for the ability to provide the high temperature heat needed by thermochemical process, and to be interfaced safely and economically to the hydrogen production process. The recommended reactor technology should require minimal technology development to meet the high temperature requirement and should not present any significant design, safety, operational, or economic issues. These considerations should be applicable to high temperature electrolysis and hybrid thermochemical cycles as well.
The reactor coolant becomes a primary consideration for determining which concepts are appropriate. All reactor types – including Generation IV – were assessed against five requirements and five criteria (see Table below).
Based on this assessment, and upon evaluation of the relative development requirements for candidate reactors, the following conclusions and recommendations were made:
- PWRs, BWRs, and organic-cooled reactors – cannot achieve the high temperatures needed
- .Alkali metal-cooled reactors – development risk due to materials concerns at the high temperatures needed. Lower temperature thermochemical cycles may open additional possibilities.
- Heavy metal and molten salt-cooled reactors – promising, but significant development needed.
- Gas-cooled reactors – baseline choice, only modest development needed for helium gas cooled reactors.
- Liquid-core reactors – significant development risk due to materials concerns at the high temperatures needed.
- Gas-core reactors – not recommended, too speculative.
The Gas Turbine-Modular Helium Reactor (GT-MHR, see NEI March 2004, p18) consists of 600MWt modules that are located in underground silos. The direct-cycle gas turbine power conversion system is located in an adjacent silo. By replacing the gas turbine system with a primary helium circulator, an intermediate heat exchanger, an intermediate helium loop circulator and the intermediate loop piping to connect to the hydrogen production plant, the GT-MHR can be changed into the ‘H2-MHR’, as shown.
Each 600MWt reactor module would produce about 200 tonnes of hydrogen per day. A single large ammonia plant used for fertiliser production could use two modules to produce the hydrogen it needs. A large oil refinery using hydrogen to ‘sweeten’ and lighten heavy crude oils for production of clean-burning gasoline and other fuels could use three H2-MHR modules to obtain its hydrogen. Thus the scale of nuclear production of hydrogen is well matched to the scale of current industrial demands for hydrogen. It would take about 160 of these 600MWt modules to provide all the hydrogen currently used by US industry. Providing hydrogen produced by nuclear power for this industrial use can provide the bridge needed to get to a where hydrogen is not only produced for the current industrial needs, but also is used extensively for transportation and other direct fuel needs in a true hydrogen economy.
Author Info:
Kenneth R Schultz, General Atomics, PO Box 85608, San Diego, CA 92186, USA; J Stephen Herring, Idaho National Laboratory, P.O. Box 1625, Idaho Falls, ID 83415, USA; Michele A Lewis, Argonne National Laboratory, 9700 South Cass Ave., Argonne, IL 60439, USA; William A Summers, Savannah River National Laboratory, Aiken, SC 29808, USA
TablesReactor selection requirements and criteria