Researchers from China’s Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) have developed an organic material to extract uranium from seawater. The material is reportedly cost-effective and has “exceptional uranium adsorption capability”. The research is published in the Chemical Engineering Journal, Volume 495 dated 1 September 2024.
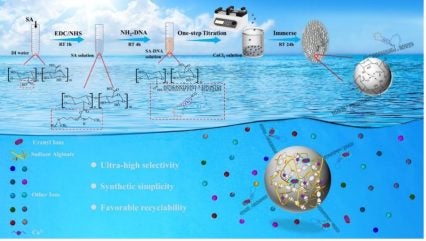
The researchers selected affordable sodium alginate (SA) and functional DNA strands to fabricate SA-DNA hydrogel microspheres for the selective adsorption of uranyl ions (UO22+) in an economically viable manner. Compared with reported advanced adsorbents utilising the amine oxime (AO) group for uranium extraction, the SA-DNA hydrogel microspheres demonstrated significantly higher selectivity for uranium.
The utilisation AO functional groups has greatly improved the efficacy of uranium extraction, and it has been regarded as the most promising approach for recovering uranium from seawater. However, the introduction of AO groups is complex and expensive. In addition, the effectiveness of these groups is significantly affected by interference from other ions, specifically vanadium ions that may bind more strongly than UO22+. Also, because these adsorbents are primarily available as powders or nanoparticles, their retrieval after extracting uranium is difficult.
The new adsorptive material is a combination of DNA enzymes, which is a specific type of DNA, and composite microspheres – a material derived from ion exchanges between sodium alginate and calcium ions. It is environmentally friendly, cost-effective, easily synthesised, and has impressive mechanical robustness and recyclability. Because of the distinct ability of specific DNA enzymes to recognise various metal ions, these adsorbents could also be used to retrieve other valuable metal ions from seawater, according to the study.
Extracting the ions has proved challenging for several reasons, including the extremely low concentrations of uranium in seawater. One tonne of seawater contains only 3.3 milligrams of uranium – and the presence of various other ions can interfere in a complex marine environment. This is like finding 1 gram of salt in 300,000 litres of fresh water.
The adsorption capacity of uranium in natural seawater was 8.62 times higher than that of vanadium, indicating substantial selectivity of the SA-DNA hydrogel microspheres for UO22+ in practical applications. The new adsorbent exhibited excellent adsorption performance under prolonged immersion in natural seawater, particularly with respect to its ultra-high selectivity for uranium.






